May 25, 2021 - Salt Lake City, Utah. - oneSOURCE Document Management Services, Inc., an...
Read More
The latest manufacturer documents on demand.

COVID-19 Vaccine Resource Page. We’re providing free, easy access to our growing collection of manufacturer documents regarding approved vaccines and related storage and other equipment to help you with regulatory requirements.
oneSOURCE is a web-based solution allowing all healthcare departments and staff easy access to Instructions For Use (IFUs), cleaning protocols, service manuals, and Safety Data Sheets (SDS).
oneSOURCE Complete: Ensure that your healthcare organization stays compliant with such regulatory bodies as The Joint Commission, CMS, AAAHC, DNV, CDC Guidelines, and OSHA, to secure Medicare reimbursements as well as limit the errors related to sterile processing, cleaning, and maintenance.
The RLDatix Compliance bundle builds off the oneSOURCE platform to take patient safety to the next level. While oneSOURCE provides healthcare staff with easy-to-access documents to answer any questions related to the cleaning and reprocessing of any device, PolicyStat takes it a step further and gives your team the ability to link those same documents to relevant policies. This allows your organization to be consistently notified whenever there are any policy or manufacturer updates, regardless of the department they’re in. Additionally, the Accreditation and Regulatory (A&R) platform provides a rounding tool that helps link policies, procedures, and documents to make your audit process easier, helping your team remain survey ready.
See below to learn more about the other products in our Compliance bundle:
Healthcare agencies and accreditation organizations must verify that important manufacturers’ product information is readily available to technicians throughout the healthcare facility.
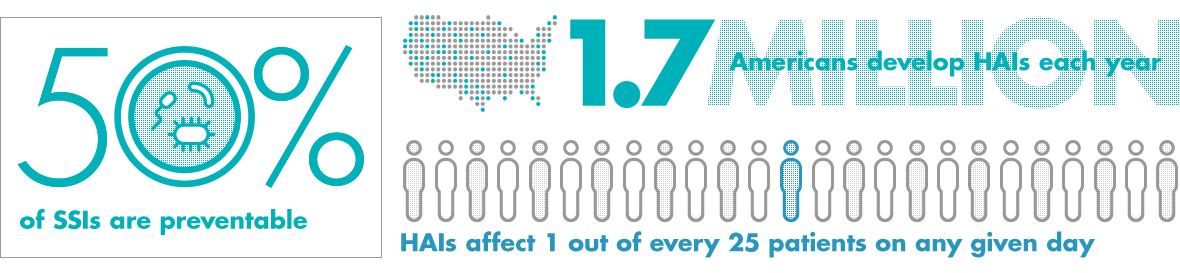
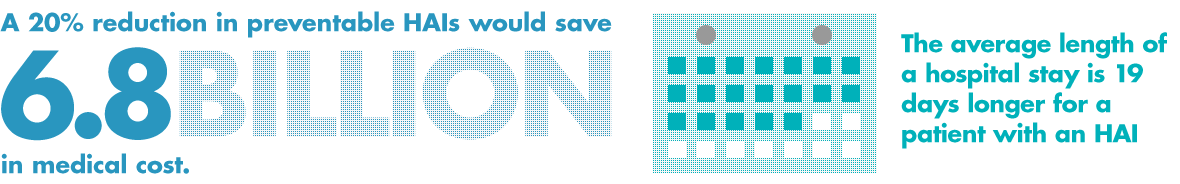
May 25, 2021 - Salt Lake City, Utah. - oneSOURCE Document Management Services, Inc., an...
Read MorePandemic punctuates off-site, on-site reprocessing decisions.
Read MoreSALT LAKE CITY (May 25, 2021) - oneSOURCE Document Management Services, Inc., an RLDatix company...
Read MoreAs members of the HTM field, you’ve likely been called one of healthcare’s “unsung heroes.”...
Read MoreMarch 10, 2021 – Salt Lake City, Utah – Today, oneSOURCE Document Management Services, an...
Read MoreFebruary 23, 2021 — PLYMOUTH MEETING, PA—ECRI, the nation’s largest patient safety and independent device...
Read MoreECRI and oneSOURCE Document Management Services have announced a joint collaboration focused on improving medical...
Read MoreECRI and oneSOURCE Document Management Services announced a joint collaboration focused on improving medical device...
Read MoreDOTmed/Healthcare Business News (169,814 UVPM) also shared the partnership with their audience: https://www.dotmed.com/news/story/54052
Read More“I was impressed with oneSOURCE document site's service even before we became customers. All our questions regarding instrument reprocessing were answered, and I received in hand the manufacturers' instruction document that day. It was amazing. Now that we are customers, oneSOURCE document site has improved the efficiency of our Sterile Processing Departments with easy-to-use, unlimited access at nine different sites.”
Sign up today to receive more information
{CSTM_post_description}